This transcript has been edited for clarity.
From 1981, when the first patients with HIV were identified, to 2007, a cure for the deadly virus would have been a miracle. Then, Timothy Ray Brown — known as "The Berlin Patient" — was cured. And just like that, a miracle became science.
Mr Brown had been diagnosed with HIV in 1995. In 2006, he was diagnosed with acute myelogenous leukemia (AML), which would require a donor bone marrow stem cell transplant for treatment. But this wasn't just any donor. His donor was one of less than 1% of the world's population, with a unique genetic mutation known as the CCR5 delta 32 mutation. This rendered the donor's immune cells resistant to HIV.
It was a gamble, to be sure, but it proved to be a successful one. Mr Brown lived from 2007 to 2020 without the need for antiretroviral therapy. He died of a relapse of AML. No HIV was detected at the time of his death.
The question, of course is simple: Why can't more people be cured of HIV?
Let's state the obvious first. Curing HIV with a bone marrow transplant is a bit like swatting a fly with a sledgehammer. In an era where antiretroviral therapies are highly effective and can lead to essentially normal life expectancy, undergoing a procedure involving high-dose chemotherapy, radiation, stem cell transplant, and lifelong immunosuppression does not exactly minimize the risk-benefit ratio.
So to date, all cases of HIV cure via bone marrow transplant — and there have been perhaps five — have been in the context of treatment for some other disease: leukemia or lymphoma.
The reason for the vanishingly small numbers is not simply that bone marrow transplants are rare. The stars really need to align for these patients because not only do they need to find a suitable donor, but they need to find one who carries the CCR5 delta 32 mutation. Take the number of eligible donors you may have for a bone marrow transplant — already a very small number — and divide it by 100.
And, by the way, if you are of non-European ancestry, your chances are even lower. People of non-European ancestry are substantially underrepresented in bone marrow registries in the first place. They are also less likely to have that particular beneficial mutation. Needle in a haystack doesn't do this justice. We're talking a needle in a field of haystacks.
But the stars might not need to align quite as perfectly anymore, thanks to research presented in this paper, appearing in Cell, that shows what appears to be the first HIV cure that is a result of cord blood-derived stem cells.
The woman described in the manuscript, being treated for leukemia, characterized herself as mixed-race, which already makes the chance of finding a suitable bone marrow donor low. But a donor was found, matching in eight out of eight haplotypes — the proverbial needle in a haystack. But, this donor, like the vast majority of us, had the normal CCR5 gene, not the HIV-resistant version.
This is where it gets clever. The researchers had been screening cord blood registries for samples with the HIV-resistant mutation. Out of 18,000 samples, they found 171 with the beneficial mutation. One of those 171 samples was a five out of eight match for the patient in this study. And that's OK — because prior research has shown that cord blood transplants fare better in the context of HLA-mismatch compared with traditional bone marrow transplants.
The patient was exposed to high-dose chemotherapy, radiation, and other treatments to eradicate her own immune cells, and given both the adult donor and the resistant cord blood samples. The researchers believed that the adult cells would support immune reconstitution as the cord blood cells slowly took over.
And they were right.
This graph shows where her white blood cells were coming from after the transplant over time. At first, the adult donor cells predominated, but by 14 weeks, her immune system was entirely repopulated by those cord blood cells — the cells that were resistant to HIV.
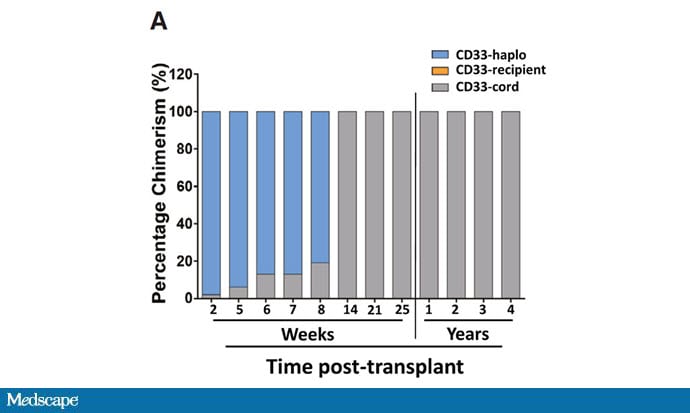
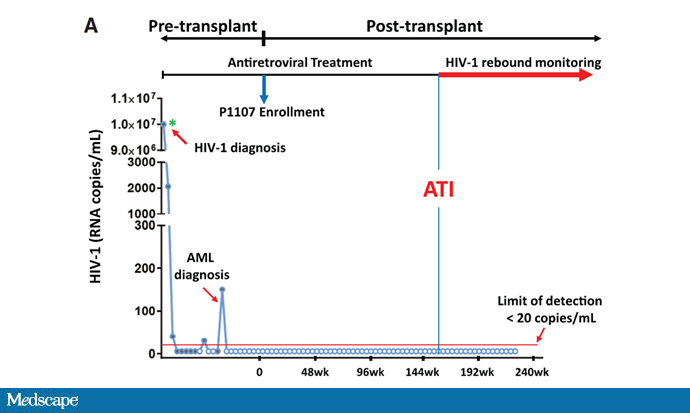
The patient recovered from the procedure and remained on antiretroviral therapy for about 3 years. Her viral load was fully suppressed throughout that time. And then, in careful discussion with her doctors, she stopped the antiretroviral therapy.

Her viral load remained suppressed. It's been 2 years, with no viral load off any anti-HIV therapy.
The researchers weren't content to only look at viral load. After all, individuals on anti-HIV therapy often have undetectable viral loads, and we don't call them cured because there are viral reservoirs in the system.
They used the quantitative viral outgrowth assay (QVOA) to search for those reservoirs. As you can see here, there weren't any.

In a petri dish, they added live HIV virus to the patient's new immune cells to see if they would get infected. Control cells were rapidly invaded. The patient's cells? Nothing.

They even tested the cerebrospinal fluid, since some studies have suggested that the nervous system can be an HIV reservoir. Nothing there either.
Of course, we might be premature calling this a cure. Time will tell how durable this remission is. But the bigger picture here is the opportunities this study opens up.
Cord blood has multiple advantages over adult-derived bone marrow stem cells. Harvesting cord blood is painless and safe. Cord blood transplants have less graft-vs-host disease. In contrast, the Be The Match bone marrow registry has 22 million individuals in it who could be called up to donate bone marrow — largely of European ancestry. A White person has a 77% chance of finding a donor in the registry. A Black person has only a 23% chance.
It's great to have 22 million people in the international bone marrow registry. But that many people are born every 2 months, and cord blood can be taken on the spot — no need to contact donors later. The possibilities for cord blood are staggering. And while it may start with the rarest of rare cases — a patient with HIV, and a cancer amenable to stem cell transplant — where it will end may be transformational.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale's Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn't, is available now.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Credits:
Image 1: Cell
Image 2: Cell
Image 3: Cell
Image 4: Cell
Image 5: Cell
Medscape © 2023 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: F. Perry Wilson. How the Stars Align to Cure HIV - Medscape - Mar 16, 2023.














Comments