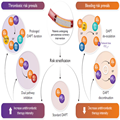
Graphical Abstract
Risk-based antithrombotic strategies after percutaneous coronary intervention (PCI). Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor is indicated for patients undergoing PCI. Applying a risk stratification approach that considers the risk of both thrombosis and bleeding, each individual patient can be managed with a tailored antithrombotic regimen. If the two risks substantially equipoise, standard DAPT is indicated (i.e. aspirin plus a P2Y12 receptor inhibitor for 6 or 12 months, based on the clinical scenario). In patients with a prevailing thrombotic risk (left part of the figure), the intensity of the antithrombotic therapy can be increased by administering DAPT for a prolonged duration or by prescribing a dual-pathway inhibition therapy (i.e. ASA plus rivaroxaban 2.5 mg twice daily). Conversely, when the bleeding risk prevails (right part of the figure), the intensity of the antithrombotic therapy can be decreased by de-escalation to a reduced intensity regimen (i.e. switching from a potent P2Y12 receptor inhibitor to clopidogrel or reducing the P2Y12 receptor inhibitor dose) or by early discontinuation of DAPT with transition to a monotherapy with either ASA or a P2Y12 receptor inhibitor. Abbreviations: ASA, acetylsalicylic acid; BID, bis in die (twice daily); C75, clopidogrel 75 mg; P2Y12-i, P2Y12 receptor inhibitor; OD, once daily; P10, prasugrel 10 mg; R2.5, rivaroxaban 2.5 mg; T60, ticagrelor 60 mg; T90, ticagrelor 90 mg.
Dual antiplatelet therapy (DAPT) is a treatment standard for percutaneous coronary intervention (PCI) with drug-eluting stents due to concerns of impaired vascular healing and stent thrombosis.[1] With these concerns now largely mitigated by advances in stent technology, the focus has shifted to bleeding, a prognostically unfavourable complication of DAPT. Several alternatives to a standard regimen of DAPT have been introduced in clinical practice to improve the efficacy and safety outcomes of PCI according to the individual risks of thrombosis and bleeding (Graphical Abstract). Given the ability of current-generation stents to heal rapidly, in particular, extending DAPT over 1–3 months might be unnecessary especially when PCI patients present with characteristics of high bleeding risk (HBR).[2] However, DAPT also confers protection at the level of non-stented coronary segments and other parts of the arterial tree. Therefore, the net benefit of shortening DAPT (i.e. reduced bleeding vs. withdrawal of thrombotic protection) is uncertain in general and mostly in patients with HBR who also present with a high risk of thrombosis.
For patients at HBR who undergo PCI for a chronic coronary syndrome (CCS) and have no indication for oral anticoagulation, guidelines from the European Society of Cardiology recommend 3 months (class IIa) or 1 month (class IIb) of DAPT with aspirin and the P2Y12 receptor inhibitor clopidogrel.[3] For corresponding patients with an acute coronary syndrome (ACS), discontinuation of the P2Y12 inhibitor prasugrel, ticagrelor, or clopidogrel at 3 months (class IIa) or discontinuation of aspirin at 3–6 months (class IIa) is recommended.[4] The level of evidence supporting these recommendations is 'A' (i.e. data derived from multiple randomized trials or meta-analyses) only for discontinuation of clopidogrel at 3 months in CCS and discontinuation of aspirin at 3–6 months in ACS. On the contrary, weaker evidence levels ('B' or 'C') support discontinuing the P2Y12 inhibitor at 3 months in ACS and discontinuing clopidogrel at 1 month in CCS. Hence, there is a need to reinforce the body of literature supporting these recommendations.
A study-level meta-analysis by Costa et al., the results of which are published in this issue of the European Heart Journal, is timely and useful for that purpose.[5] The study pooled 9006 HBR patients from 11 trials of abbreviated (1–3 months) vs. standard DAPT (≥6 months), the majority of whom had an ACS (58% of patients) and non-complex PCI characteristics. The risk of the primary bleeding endpoint (a composite of major or clinically relevant non-major bleeding at 12 months) was significantly reduced with the abbreviated DAPT regimen (risk ratio, 0.76; 95% confidence interval, 0.61–0.94), which did not modify the risk of major adverse cardiac events (MACE) based on two definitions (i.e. the composite of death, myocardial infarction, or stroke and the composite of cardiovascular death, myocardial infarction, or stroke). Importantly, there was no apparent interaction between clinical presentation and the treatment effect of abbreviated DAPT on bleeding and MACE, meaning that the benefit of shortening DAPT applied equally to HBR-PCI patients with CCS or ACS.
A major strength of this meta-analysis regards its collaborative nature since unpublished data were made available by the investigators of individual trials to censor events occurring at times when the treatment strategies did not diverge (i.e. in trials that randomized patients at the time of PCI rather than after an initial period of DAPT). However, a meta-analysis based on individual patient data would have elevated this collaboration to an even higher level, enabling more analyses and insights. The only trial that specifically randomized HBR patients was MASTER DAPT,[6] while the other studies included HBR and non-HBR patients and did not stratify the randomization by HBR status. As such, the comparison of abbreviated vs. standard DAPT in the pooled HBR subgroup does not exclude the presence of potential confounders; again, an individual patient data meta-analysis would be the best setting to eventually identify such confounders and address them with statistical adjustment.
These strengths and limitations being acknowledged, is the evidence provided by the meta-analysis of Costa et al. a compelling reason to justify a stronger grade of recommendation and level of evidence for 1–3 months of DAPT after PCI in CCS or ACS patients with HBR? To answer this question, one must keep in mind the size of the sample required to conclusively accept or reject the presence of a significant difference for a given outcome. In the standard DAPT arm, the meta-analysis reported an incidence of ~7% of major or clinically relevant bleeding at the mean follow-up of the included trials (323 events in 4530 patients). Assuming a type I error of 5%, a power of 80%, and a clinically significant reduction in relative risk of 20% with abbreviated DAPT, ~9500 patients would be required to establish the superiority of this approach. With 9006 patients in the meta-analysis, the information size collected so far on this topic is reassuring. Indeed, there is little contention that bleeding is mitigated when one antiplatelet drug is used rather than two. Major bleeding carries important prognostic consequences that also relate to disruption of antiplatelet therapy and withdrawal of protection; therefore, avoiding bleeding is an essential component of optimal care.[7] In the meta-analysis, the reduction in bleeding with abbreviated DAPT was not only biologically plausible but also large in magnitude, corresponding to a number of HBR patients to treat to avoid one major or clinically relevant non-major bleeding of 41. When the HBR definition of the Academic Research Consortium was used instead of dichotomization by the PRECISE-DAPT score, this result was relatively consistent, with a number needed to treat of 45.
That said, the rationale of such types of meta-analyses is mainly to increase the precision around thrombotic and/or other infrequent endpoints. Indeed, shortening DAPT may expose patients to some withdrawal of protection, and reducing bleeding is not enough if MACE are found to increase in parallel. The raw incidences of the two MACE endpoints with standard DAPT at the mean follow-up of the included trials were ~7% and ~6%, respectively. With non-significant risk ratios of 0.97 and 0.92 with short DAPT, it is unlikely that a meaningful difference in these endpoints will be demonstrated by conducting additional trials. As regards cardiovascular death and all-cause death, more data are theoretically necessary to exclude a significant difference for these endpoints, although the trend observed in the meta-analysis goes in the direction of neutrality or possible benefit. Indeed, based on the risk of cardiovascular death or all-cause death estimated for patients on prolonged DAPT in the meta-analysis (~4% and ~3%, respectively), the sizes required to reach conclusive statements of superiority for short DAPT on these outcomes are as large as ~17 000 and ~23 000 patients, respectively. This is noteworthy because the finding of a 21% relative risk reduction in cardiovascular mortality with short DAPT in the meta-analysis comes from a pooled patient sample that is prone to the possibility of false-positive findings for rarer endpoints.
In general, the results of the meta-analysis of Costa et al. suggest that short DAPT reduces bleeding in patients with HBR-PCI and does not harm them. When applying the results of this study to current practice, one must keep in mind that patients with oral anticoagulation were excluded, and PCI complexity was not high. For patients undergoing non-complex PCI who are at HBR but without an indication for chronic anticoagulation, it is fair to say that 1–3 months of DAPT reduces the risk of major or clinically relevant non-major bleeding, without apparently increasing the risk of thrombotic events. With a dedicated randomized trial (MASTER DAPT) and now a larger meta-analysis supporting this statement, the level of evidence for short DAPT in HBR patients qualifies for the 'A' stamp. Issuing a class I of recommendation would also not be inappropriate if the phrasing of the recommendation is specific to HBR patients where concerns of bleeding outweigh the concerns of thrombosis. Identifying such HBR patients can be aided by dedicated tools[8–11] but is ultimately within the scope of clinical judgement and the art of medicine.
Eur Heart J. 2023;44(11):969-971. © 2023 Oxford University Press
Copyright 2007 European Society of Cardiology. Published by Oxford University Press. All rights reserved.