Abstract and Introduction
Introduction
The 2022–23 influenza season shows an early rise in pediatric influenza-associated hospitalizations.[1] SARS-CoV-2 viruses also continue to circulate.[2] The current influenza season is the first with substantial co-circulation of influenza viruses and SARS-CoV-2.[3] Although both seasonal influenza viruses and SARS-CoV-2 can contribute to substantial pediatric morbidity,[3–5] whether coinfection increases disease severity compared with that associated with infection with one virus alone is unknown. This report describes characteristics and prevalence of laboratory-confirmed influenza virus and SARS-CoV-2 coinfections among patients aged <18 years who had been hospitalized or died with influenza as reported to three CDC surveillance platforms during the 2021–22 influenza season. Data from two Respiratory Virus Hospitalizations Surveillance Network (RESP-NET) platforms (October 1, 2021–April 30, 2022),§ and notifiable pediatric deaths associated¶ with influenza virus and SARS-CoV-2 coinfection (October 3, 2021–October 1, 2022)** were analyzed. SARS-CoV-2 coinfections occurred in 6% (32 of 575) of pediatric influenza-associated hospitalizations and in 16% (seven of 44) of pediatric influenza-associated deaths. Compared with patients without coinfection, a higher proportion of those hospitalized with coinfection received invasive mechanical ventilation (4% versus 13%; p = 0.03) and bilevel positive airway pressure or continuous positive airway pressure (BiPAP/CPAP) (6% versus 16%; p = 0.05). Among seven coinfected patients who died, none had completed influenza vaccination, and only one received influenza antivirals.†† To help prevent severe outcomes, clinicians should follow recommended respiratory virus testing algorithms to guide treatment decisions and consider early antiviral treatment initiation for pediatric patients with suspected or confirmed influenza, including those with SARS-CoV-2 coinfection who are hospitalized or at increased risk for severe illness. The public and parents should adopt prevention strategies including considering wearing well-fitted, high-quality masks when respiratory virus circulation is high and staying up-to-date with recommended influenza and COVID-19 vaccinations for persons aged ≥6 months.
CDC collects data on influenza-associated hospitalizations through the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based RESP-NET system that includes more than 250 acute care hospitals.§§ Since March 2020, CDC has also collected data on COVID-19–associated hospitalizations through another RESP-NET platform, the COVID-19–associated Hospitalization Surveillance Network (COVID-NET). Influenza and SARS-CoV-2 testing¶¶ is driven by clinician decisions or hospital testing policies, with laboratory, clinical, and notifiable disease database sources used to identify patients.*** A FluSurv-NET patient was defined as a person who 1) was a resident of the surveillance catchment area, 2) had a hospital admission during October 1, 2021–April 30, 2022, and 3) had a positive influenza test result within 14 days before or anytime during hospitalization. Coinfected patients were those who met the FluSurv-NET definition and who also 1) had laboratory-confirmed influenza and SARS-CoV-2 infections during the same hospitalization, or 2) were identified through COVID-NET and had a COVID-19–associated hospital admission occurring within 14 days before or after an influenza-associated hospitalization. A patient was considered to have received the current seasonal influenza vaccine if ≥1 dose was administered ≥14 days before the positive influenza test result.†††
Data on influenza-associated pediatric deaths that occurred during October 3, 2021–October 1, 2022, were obtained from the Influenza-Associated Pediatric Mortality Surveillance System. A notifiable death is defined as a death in a person aged <18 years resulting from a clinically compatible illness confirmed to be influenza by laboratory testing§§§ without a period of complete recovery between illness onset and death. State and local health departments report investigations of these deaths to CDC using a standardized case report form, which includes data on demographic characteristics, influenza testing, bacterial and viral co-detections, clinical diagnoses and complications, medication use, and influenza vaccination. Coinfections with SARS-CoV-2 were identified using the "viral coinfection" field, with either COVID-19 or SARS-CoV-2 indicated in free text.
Across all data sources, patients were eligible to be included in this analysis if they were aged <18 years and had evidence of influenza virus infection. Information on COVID-19 vaccination and antiviral treatment was not included because of lack of systematic ascertainment for patients across data sources. Demographic and clinical characteristics, in-hospital interventions, and outcomes are reported by illness status (influenza and SARS-CoV-2 coinfection and influenza infection alone) as frequencies and proportions, with between-group comparisons analyzed using Pearson's chi-square tests for hospitalizations and Fisher's exact tests for deaths. Medians and IQRs are presented for continuous variables, with between-group comparisons analyzed using a Wilcoxon rank sum test. Data were analyzed using SAS software (version 9.4, SAS Institute). These activities were reviewed by CDC and were consistent with applicable federal law and CDC policy.¶¶¶
Hospitalizations
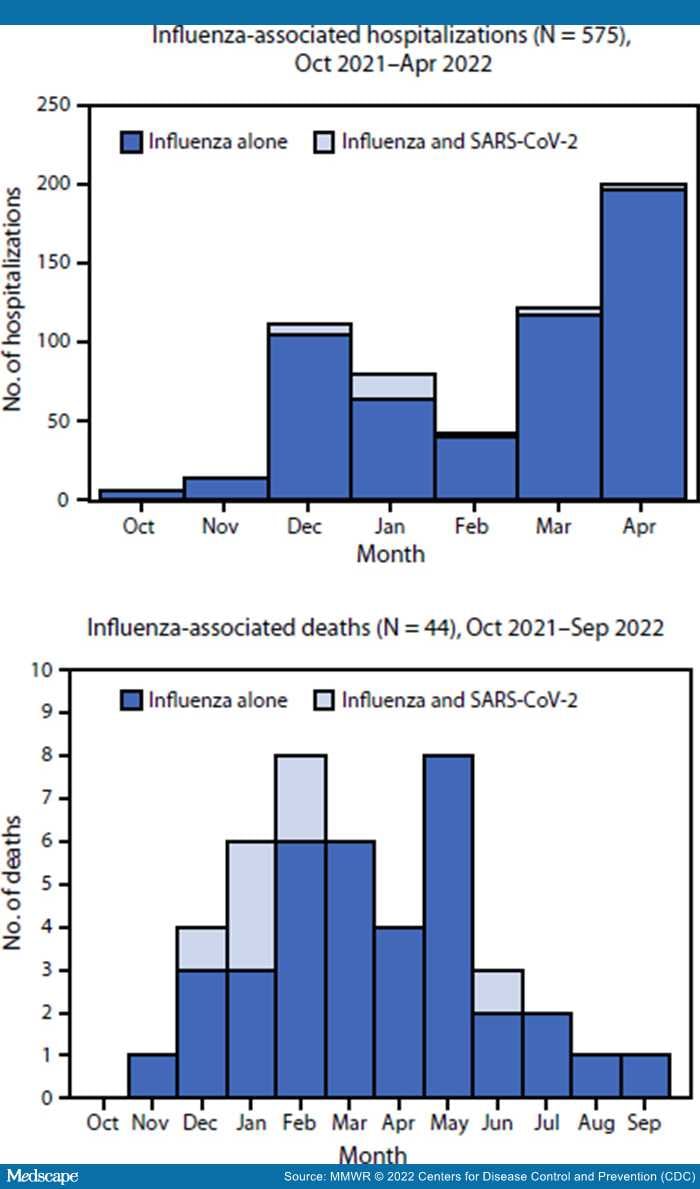
During October 1, 2021–April 30, 2022, FluSurv-NET identified 575 pediatric influenza-associated hospitalizations, including 32 (6%) patients who were coinfected with SARS-CoV-2 and 543 (94%) who had influenza alone (hereafter, influenza) (Figure). Underlying medical conditions were reported for the majority of hospitalized patients with coinfection (56%) and with influenza (58%) (p = 0.81), whereas receipt of seasonal influenza vaccination was less prevalent among those with coinfections (17%) than among those with influenza (42%) (p = 0.02) (Table 1). A higher proportion of patients with coinfection than with influenza received invasive mechanical ventilation (13% versus 4%; p = 0.03) and BiPAP or CPAP (16% versus 6%; p = 0.05). No significant differences were found between patients with coinfection and with influenza in the prevalence of intensive care unit (ICU) admission (p = 0.20). No in-hospital deaths were identified with FluSurv-NET in either group.
Figure.
Number of children and adolescents aged <18 years who were hospitalized* or died† with influenza alone and influenza and SARS-CoV-2 coinfections, by month — United States, 2021–22 influenza season
*Influenza Hospitalization Surveillance Network; data beyond April 30, 2022, did not include variables required to determine influenza and SARS-CoV-2 coinfection status.
†Influenza-Associated Pediatric Mortality Surveillance System.
Deaths
Forty-four influenza-associated pediatric deaths were reported to the Influenza-Associated Pediatric Mortality Surveillance System during the 2021–22 influenza season, including seven (16%) decedents who had SARS-CoV-2 coinfection (Figure). Among influenza vaccine–eligible children who died and for whom data were available, zero of six with coinfections and five (16%) of 31 with influenza had been vaccinated against influenza (p = 0.57) (Table 2). The most common complications among decedents with coinfections were pneumonia, acute respiratory distress syndrome, and bronchiolitis. Among decedents with influenza, the most common complications were pneumonia, seizures, and acute respiratory distress syndrome. Cardiomyopathy or myocarditis occurred in five (16%) of 32 decedents with influenza and none with coinfection (p = 0.57). One or more underlying medical conditions were reported for four of five children with coinfections who died and 21 (58%) of 36 with influenza (p = 0.63). Influenza antiviral therapy was administered to one child with a coinfection who died and 17 (46%) decedents with influenza (p = 0.21).
Morbidity and Mortality Weekly Report. 2022;71(50):1589-1596. © 2022 Centers for Disease Control and Prevention (CDC)