This article was originally published November 6 on Medscape editor-in-chief Eric Topol's substack "Ground Truths."
Last December I wrote a piece here on Paxlovid, "a just-in-time breakthrough" that went through the mechanism of the drug, the results of the randomized, placebo-controlled clinical trial in high-risk individuals subsequently published in the New England Journal of Medicine (below), and why, because its effect was independent of new variants, that the timing of its documented high efficacy (just when Omicron hit) was ideal. An 89% reduction of hospitalizations and deaths by day 28 (absolute reduction of 6.2/100) was impressive, along with the finding of 0 deaths in the Paxlovid group (7 in the placebo arm). Viral load was rapidly and significantly reduced with treatment. There were less side effects in the Paxlovid than placebo arm.
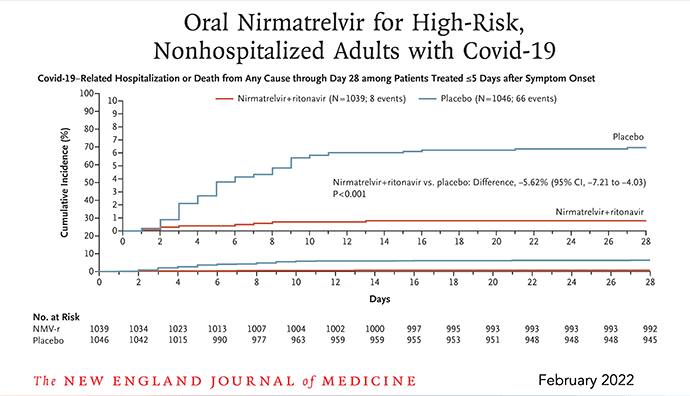
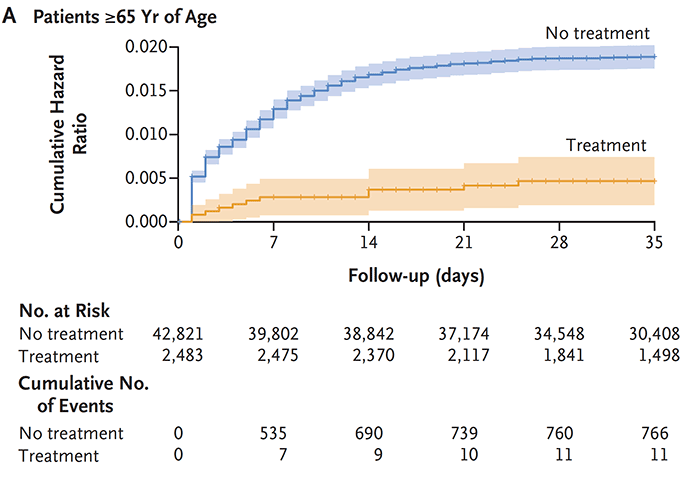
Subsequently, there have been many clinical effectiveness studies throughout the world which have not only confirmed this high level of protection from hospitalizations and deaths, but many, such as this one from Israel, showed the partitioning of acute phase benefit by age, not seen in people younger than age 65.
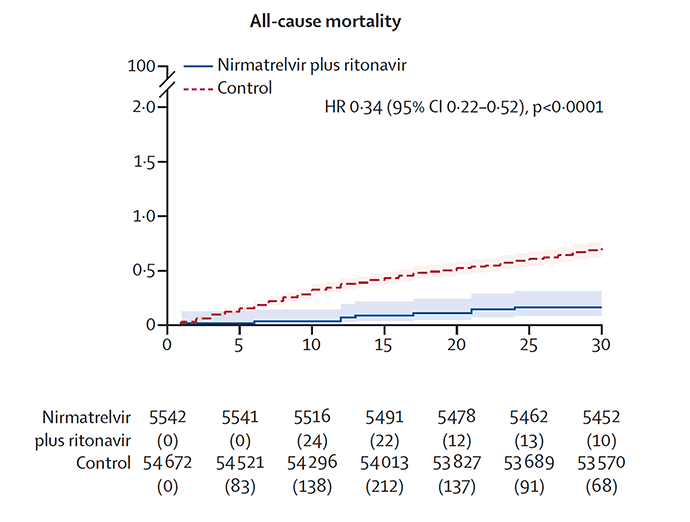
There was also the Hong Kong matched pair study of Paxlovid indicating a 66% reduction of all-cause mortality in people age 65 and older (just 17% were < age 65 in that study). Side note: we don't have many interventions in medicine that achieve this level of proportionate all-cause mortality reduction.
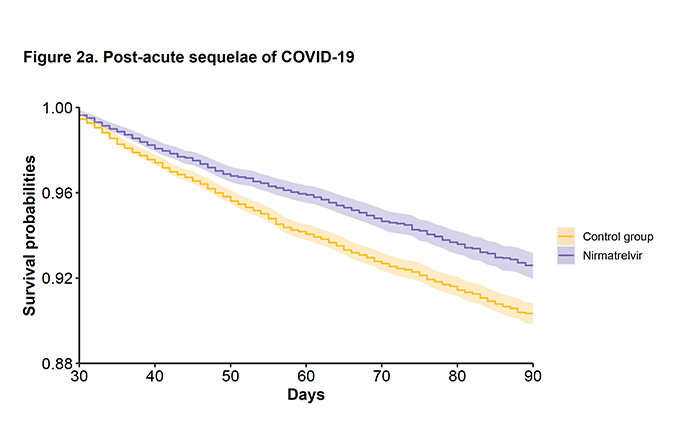
All of this efficacy was in the first month after Paxlovid therapy. We really had no idea whether the treatment would have subsequent impact. Until now.
The New VA Study
A new report from the Veterans Affairs health system (now in preprint), the largest in the United States, is the first study to look at longer term effects. Dr. Ziyad Al-Aly and colleagues, who have published many of the most important papers on Long Covid in leading peer review journals, now studied over 9,000 paxlovid treated patients (within 5 days of symptom onset; in March-June 2022 during Omicron and subvariant waves; the sample size is considerably larger than the 3 studies above) and compared the results to ~47,000 controls. Their mean age was 65 years and 12% were female. The results of reducing the toll of Long Covid, its complications, and extending benefit of survival and avoidance of hospitalizations out to 1-year were all notable.
Here is the graph of the occurrence of Long Covid over time, a 26% reduction for Paxlovid. The curves are continuing to diverge over time so it will be quite interesting to see more extended follow-up.
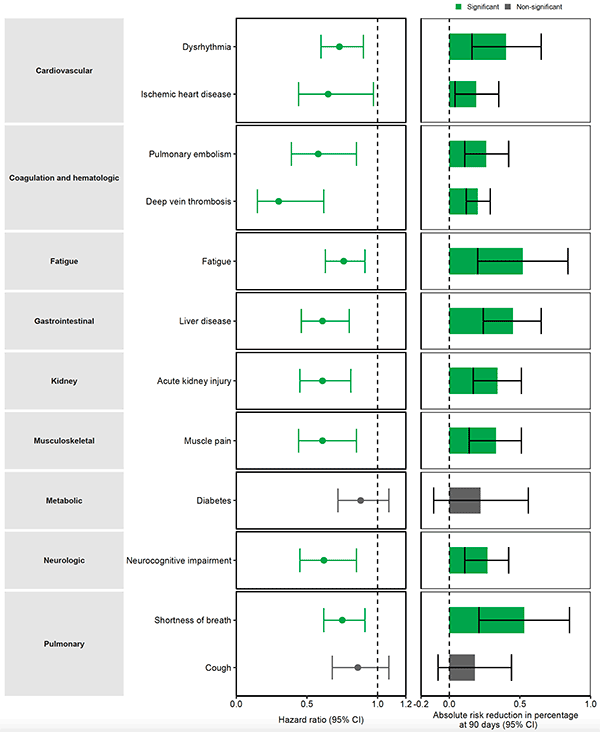
That was broken down into 12 components (by systems and symptoms) and all but 2 were significantly reduced by Paxlovid. The relative reduction is on the left, the absolute reduction at right.
Of particular note is the further reduction of deaths and hospitalizations after the first month of treatment. You can see the curves diverging here, more than they were by 1 month.
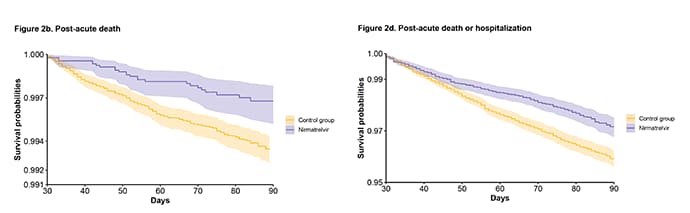
This represents a 48% reduction of death and 30% reduction in hospitalizations after the acute phase.
It is important to highlight that there was no difference noted by vaccination or booster status, prior infection, or unvaccinated status. The benefit was the same by sex of the patients. Although the mean age was 65 years, the benefit for patients less than age 60 was the same as greater than 70. The number of baseline risk factors or comorbidities did not influence the magnitude of benefit.
Now Three Ways to Prevent Long Covid?
Before this report, the only way we have known to reduce Long Covid was to avoid a Covid infection (100% effective!) and some reduction afforded by prior vaccination and boosters, the level of that protection mostly in the range of 30 to 50%. The new VA study adds a 3rd way of reducing Long Covid, no less important complications across multiple organ systems, and improved survival and avoidance of hospitalizations. It is the first such report, so it will need to be independently replicated to be certain it holds up. Let's assume for now that it will. Why did it work?
Potential Mechanism of Benefit
We know a major underpinning of Long Covid consists of the enduring viral reservoir and remnants, as documented by multiple studies, along with auto-immunity directed at the persistent virus or virus particles. Paxlovid stops replication of the virus via inhibiting its main protease, such that the likelihood of the virus establishing a reservoir or leaving behind remnants would be reduced.
There have been concerns that people who are vaccinated would not mount an immune response if they received paxlovid early in the course of a breakthrough infection, which has been linked to the concerns of rebound. The current study results point against this concern, since the vaccinated and boosted patients did just as well — they derived equal benefit — as unvaccinated, in long-term follow-up. Our group and others will soon be presenting data that the rebound (positive test with or without symptoms) after paxlovid, adjusting for rebound that occurs without Paxlovid, is infrequent, in the 10% ballpark, when systematically studied.
The Age Issues
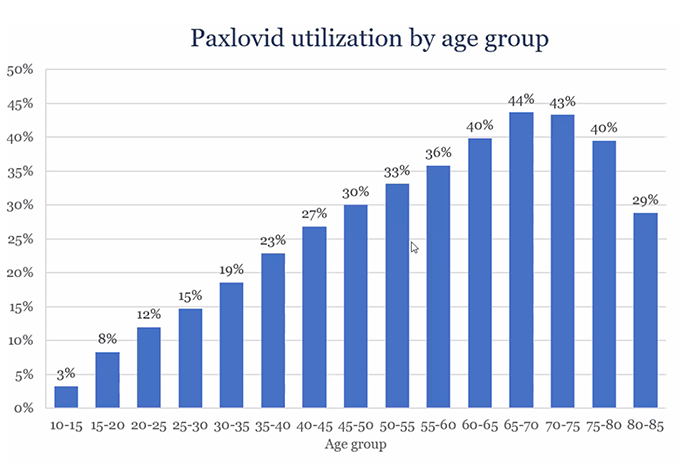
In the United States, there is a marked decline the use of Paxlovid for people age 70+. As seen in this graph, a person age 45-50 is as likely to get treated as one 80+. The reasons for that are not entirely clear, but concerns about interactions with medications is likely high on the list. Nearly all of the interactions can be safely avoided by stopping the medication for the brief 5-day period of paxlovid treatment.
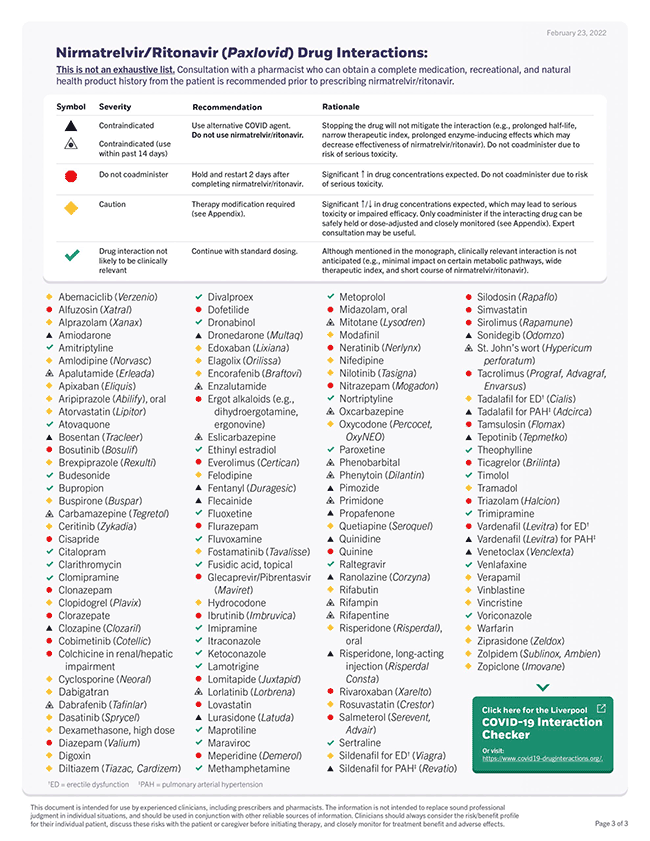
There are many Paxlovid drug-interaction checkers, but the one I find most useful is from the University of Liverpool summarized in the Table below. As you'll see there are very few concurrent medications that are contraindicated (black triangle). For most, the interactions can be avoided by holding the medication of concern until the 5-day treatment course is completed.
That issue applies primarily to older patients who may be candidates for Paxlovid. What about younger? We don't have much data to show acute phase benefit for people less than age 60, and the protection from Long Covid in the new VA study had a limited number of people age <60, even though the subgroup result was encouraging. This represents a current hole in the data, for it is possible/likely that the acute phase benefit in the younger age groups is minimal (with low acute phase risk), but reduction of Long Covid, which mostly occurs in younger people, age 30-50 years, is equal to (or potentially greater) than the older age groups.
Paxlovid to Treat Long Covid?
Unfortunately, we have no treatment that has been validated to treat Long Covid. Rigorous, randomized, definitive clinical trials are long overdue. In August 2022, Nature had a feature culling together 26 small clinical trials and paxlovid was on the list. But all of these trials are too small to provide adequate evidence, as I previously reviewed. There are several case reports, anecdotal evidence of potential benefit of paxlovid in people with Long Covid. Finally, the National Institutes of Health is planning to launch a trial to start in early 2023 of 1,700 participants age 18+ with Long Covid. That sample size is quite small relative to the burden of Long Covid, but the results, not expected until 2024, will provide some important new evidence for this key question along with a study among younger participants.
Bottom Line
It's exciting to see a potential 3rd path to prevent/reduce the risk of Long Covid. There are uncertainties such as the younger age benefit, and the possibility that over time we could see resistance to Paxlovid — naturally occurring mutations in the main protease with in lab confirmation of resistance have already been identified.
Nevertheless, no resistance in the clinic at any scale has thus far been documented and let's hope it stays that way. Whatever the precise mechanism of benefit, resistance to the drug will not help the cause. As with any observational study, we're taking about “association” and not cause and effect. That's why an independent replication of the durable and amplified benefit of Paxlovid versus controls over time, complementing the current VA study, will be important to see.
Let me also be clear. My interpretation of the results reviewed here is not associated with any relationship with Pfizer. I have none, and as previously reviewed here at the end of that post, and the fact I have taken this company on many times throughout the pandemic. Most recently, that Pfizer has had annual revenues of $34 billion in vaccines and $22 billion of Paxlovid and are dramatically raising the price of their boosters ~4-fold due to “weaker demand.” Sure, Pfizer has made pivotal contributions for preserving human health and saving lives during the pandemic and deserves major credit for that. But I do not see how price gouging should be an outgrowth of their success.
Last point. Until we have more data, I believe the body of evidence should make us treat more with Paxlovid to derive both acute and longer-term benefits. I have not had Covid, but if I do get it, I will be certain to take Paxlovid, temporarily stop my statin for 5-days. I will also be treating my patients with Paxlovid more liberally with knowledge of the new report.
I hope this review is helpful. Thanks for reading, subscribing, and spreading the word to others.
Credits:
Lead image: Bizoon/Dreamstime
Image 1: N Engl J Med 2022; 386:1397-1408
Image 2: N Engl J Med 2022; 387:790-798
Image 3: Lancet Inf Dis. Published online August 24, 2022
Image 4: Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19 Preprint posted November 5, 2022
Image 5: Sequelae of COVID-19 Preprint posted November 5, 2022
Image 6: Sequelae of COVID-19 Preprint posted November 5, 2022
Image 8: University of Liverpool.
Medscape © 2022 WebMD, LLC
Send comments and news tips to news@medscape.net.
Cite this: Paxlovid and Long COVID - Medscape - Nov 08, 2022.








Comments